Chemistry and Physics: An Interconnected Relationship
Written on
Chapter 1: The Nature of Science
Both chemistry and physics are integral branches of science, yet they often appear to be at odds with each other. A common question arises: why does chemistry seem to have numerous rules and exceptions, while physics appears more straightforward? As a physicist, I admit my perspective may be skewed, but let's delve into this topic.
One notable “exception” in chemistry is the octet rule, which suggests that atoms tend to have eight electrons in their outer shell. However, this isn't universally applicable; there are scenarios where atoms have fewer or more than eight electrons, or even an odd count.
To understand the relationship between chemistry and physics, it’s crucial to recognize their shared foundation in the scientific method—building and testing models. The octet rule serves as a model that helps explain atomic interactions and molecular formation.
Section 1.1: Models in Physics
Does physics also have its own “exceptions”? While they may not be termed as such, there are indeed physics models that are not flawless. For instance, consider the gravitational force on a 1-kilogram mass near the Earth's surface. The force experienced is calculated as m*g, where g equals approximately 9.8 Newtons/kilogram. However, this model is only accurate close to the Earth’s surface, and a more sophisticated model is required at greater distances.
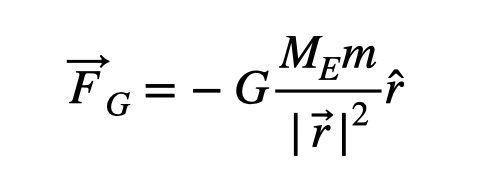
Here, we have a refined model, but it doesn't qualify as an exception.
Subsection 1.1.1: The Complexity of Atoms
Now, let's discuss why chemistry has so many exceptions; the answer lies in the complexity of atoms. We can only accurately model the simplest atom—hydrogen, which consists of one proton and one electron. For any atom more complex than hydrogen, deriving the wave function analytically becomes unfeasible.
Instead of relying solely on quantum mechanics, chemistry often employs an experimental approach. Imagine a box with buttons and lights; pressing a button activates a light, and various combinations yield different results. This trial-and-error method is akin to how chemists explore the properties of elements, as exemplified by the periodic table, which was developed by observing patterns rather than through quantum mechanical calculations.
Section 1.2: The Button-Pushing Approach
Ideally, we would dissect this metaphorical button-light box to understand its mechanics (the physicist's approach), but it may be impractical or overly intricate. This button-pushing methodology inevitably leads to exceptions, similar to the octet rule: for instance, “pressing the red button activates the red light, unless the green button is also pressed.”
Chapter 2: The Need for Both Disciplines
As we move forward, it's evident that both chemistry and physics are essential. Currently, we lack solvable models for intricate atoms and cannot create comprehensive models for complex molecules. Thus, the button-pushing strategy remains a valuable tool for discovery.
In the first video, "Science at Home #35 - Physics Vs Chemistry: Sworn enemies!!," the interrelation between physics and chemistry is explored, showcasing their distinct yet complementary roles in science.
The second video, "Connecting Physics and Chemistry in a Different Way," dives into the unique methodologies that define both disciplines and their shared quest for understanding the natural world.