Understanding the Foundations of Organic Chemistry
Written on
Chapter 1: A Challenging Introduction to Organic Chemistry
For many, the mention of "chemistry" can induce a wince. You might recall an undergraduate course that left your mind clouded with formulas and concepts you quickly forgot. Fear not—this series aims to shift that perspective, making the subject more relatable and enjoyable.
While pioneers like Davy and Faraday were busy experimenting with voltaic piles and shocking themselves, significant advancements were being made in the study of carbon-based compounds, which we now recognize as "organic" chemistry. But what led to the label "organic"? And why is carbon at the center of this field?
Consider how the early chemists were misled by the four classical elements, leading to misguided theories like phlogiston. A similar misbelief, known as "vitalism," suggested that compounds derived from living organisms could only originate from those entities and could not be synthesized artificially. Take soap, for instance: it was derived from animal fat, and chemists of the time believed that while its components could be manipulated, they could not be created by human hands due to an elusive "life force" required for their formation.
This notion was debunked in 1828 when Friedrich Wöhler synthesized urea—a substance previously extracted from urine—entirely "without the use of kidneys" (his own words!). This process is now known as the "Wöhler Synthesis." Although vitalism was dismissed, the term "organic" persisted, evolving to denote the study of carbon-containing compounds.
Organic Chemist's Perspective: Every time I see products labeled as "organic," I can't help but feel exasperated. Yes, that carton of milk contains carbon—so, naturally, it's organic! All milk is organic! If it weren't, we'd likely face dire consequences!

Chapter 2: The Scientific Landscape of Early Chemistry
Before delving deeper, let's contextualize the scientific environment of the time. Dalton had recently introduced the concept of the atom, igniting fierce debates over atomic weights. Depending on your instructor, you might have learned that carbon's atomic weight was 12 or 6, and oxygen's was 8 or 16. This inconsistency surely baffled many attempting to replicate experiments.
Modern organic chemistry's principles seemed at odds with the dualist view of chemical combinations prevalent then. However, it’s crucial to understand the scientific mindset of that era. Davy and Faraday demonstrated that compounds were fundamentally electrostatic and quasi-binary, consisting of cations (positively charged ions) and anions (negatively charged ions). The eminent organic chemist Jöns Jakob Berzelius, a foundational figure in the field, proposed that the organic part of a molecule—namely, the part containing carbon and hydrogen—could be classified as a “radical,” thus maintaining the dualistic perspective. But what exactly is a radical?
In this context, "radical" didn't imply something extreme or far-out; it referred to a fragment of a molecule considered the "root" of the compound, a concept established by Lavoisier and his students. If you have some chemistry background, you might compare a radical to a modern-day functional group. From Lavoisier to Berzelius and Wöhler, the notion persisted that these radicals were distinct clusters of atoms that acted like elements and were immutable. This classification approach was more about organizing organic compounds than accurately portraying their nature—although the two concepts are intricately linked.
Berzelius's journey was marked by humble beginnings in Sweden; after losing both parents at a young age, he was raised by relatives. Despite this, he received a robust education and initially pursued medicine. He later transitioned into full-time chemistry research, thanks in part to a sponsorship from a wealthy benefactor. His proficiency in English, German, and French kept him informed of the latest scientific developments. Berzelius meticulously developed the radical theory of organic chemistry, integrating elements of Dalton's atomic theory, and was a staunch empiricist, prioritizing observations over preconceived notions.

Chapter 3: The Evolution of Chemical Understanding
However, radical theory faced challenges. The emergence of isomers—compounds with identical atom types and counts but distinct behaviors—highlighted the limitations of this theory. For instance, consider propanol and isopropanol: both contain three carbon atoms and seven hydrogen atoms, yet they exhibit different boiling points (isopropanol at 83 °C and propanol at 97 °C). While today we understand that these differences stem from atomic connectivity and structure, early 19th-century chemists were unaware of this connection. The idea that molecular structure influences physical properties was hinted at, but Berzelius was committed to aligning radical theory with dualism, making it challenging for him to adapt.
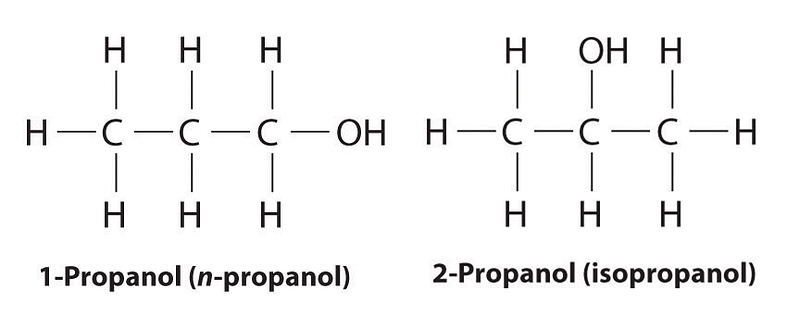
As Berzelius grappled with isomers—a term he coined in 1830—his theory struggled to explain the diversity of organic compounds compared to metallic ones. This realization underscored the significance of structural analysis in organic chemistry, marking it as a key focus moving forward. The complexities of the dualistic viewpoint were further complicated by discoveries showing that substitutions could happen within the radical itself. Berzelius contended that atoms merely rearranged, but other chemists demonstrated that hydrogen atoms in a radical could be substituted with chlorine atoms, leading to the emergence of a new classification method known as "type" theory.
This chaotic period, which lasted from around 1810 to 1860, was nonetheless productive. The discoveries made were essential for grasping the true nature of organic molecules. To achieve this ultimate understanding, chemists needed to delve into molecular structure, which became the focal point of research during this era. Additionally, organic chemists emerged as the leading analytical experimentalists of their time, reminiscent of the alchemists of the 19th century—albeit without the mystical elements.
The first video titled "Don't Let Organic Chemistry Prevent You from an Amazing Career in Medicine" by Dr. Suhaib Chaudry discusses how to overcome the challenges of organic chemistry, offering insights and encouragement for pre-med students.
The second video, "From C to A in Organic Chemistry at Stanford | The Pre-Med Playlist," provides tips and strategies for excelling in organic chemistry, essential for aspiring medical students.