Exploring the Ocean as a Vast Electrolytic Battery
Written on
Chapter 1: Introduction to Electrochemistry
My fascination with electrochemistry has persisted for quite some time. While it may be considered a niche interest, it captivates me. Specifically, my focus lies in generating hydrogen fuel from water.
As I delved into electrolysis and electrochemistry, I realized that what appears to be a straightforward process, one that an average adult could replicate at home, is, in fact, a complex phenomenon impacting our world daily.
Let's break this down. Here’s a basic design for an electrolysis cell that you can create at home. It requires the following materials:
- A non-conductive container, preferably made of glass or plastic.
- Distilled water.
- Electrodes crafted from a conductive material (preferably inert, such as stainless steel). In the diagram below, two pencils are utilized, as carbon/graphite serves as a conductor.
- An electrolyte to facilitate ion transfer for current flow.
- A DC power source, such as a 12V battery or charger.
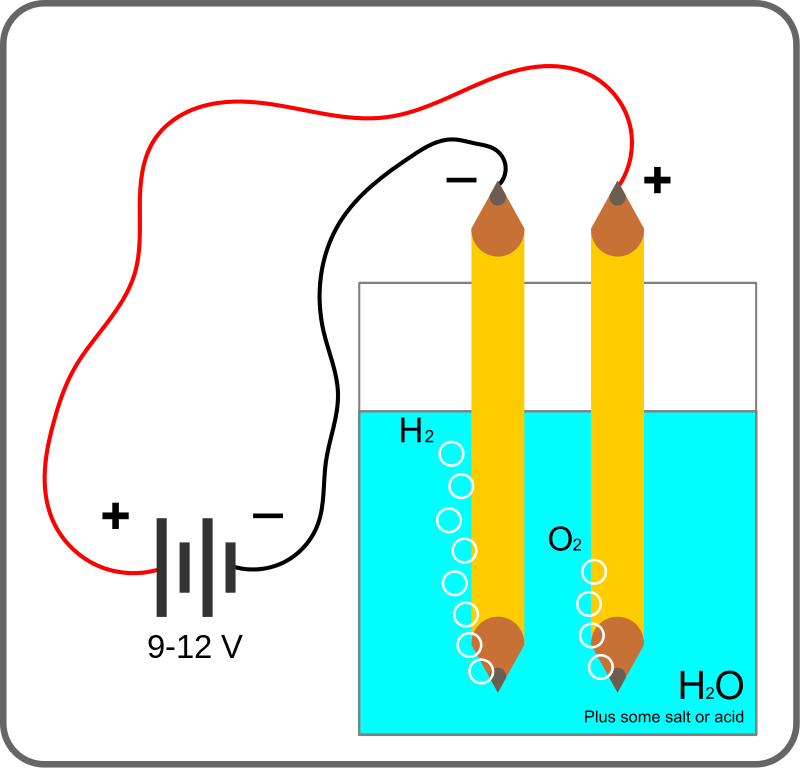
When the power source is connected to the cell (which contains equal positive and negative charges and is electrically neutral until an external force alters this balance), electrons travel to the cathode (the negative electrode). There, a chemical reaction occurs as positively charged H+ ions capture electrons and form H2 gas, which then bubbles to the solution's surface.
Simultaneously, to maintain charge balance, negative ions in the solution (OH-, if sodium hydroxide is used as the electrolyte) release electrons. These electrons enter the anode, where O2 is produced and also rises to the surface. If the electrodes are inert, they do not engage in the chemical reactions occurring within the electrolysis cell.
Chapter 2: The Ocean's Role in Electrolysis
With abundant water and many other components, including significant salt content, the oceans present an intriguing scenario. Water itself is an electrical insulator, while salt, composed of Na+ and OH-, enables electrical current flow.
This leads me to ponder whether the ocean functions similarly to an electrolysis cell. It seems plausible since lightning frequently strikes the ocean. For a visual representation, you can view a real-time map of global lightning strikes; it indicates numerous strikes occurring over oceanic regions rather than land.

To trigger a lightning strike, a voltage difference between two points is necessary. An article discusses how electrical charges are believed to form in clouds. Within these clouds, both positive and negative charges are present, allowing electrical charges to develop between areas of water and electrolytes during lightning strikes.
Notably, the upper parts of clouds typically carry positive charges, while the lower sections are negatively charged. This is crucial as we consider Earth, which might serve as an electrical generator or, at the very least, as a reservoir of electrical energy, akin to a capacitor.
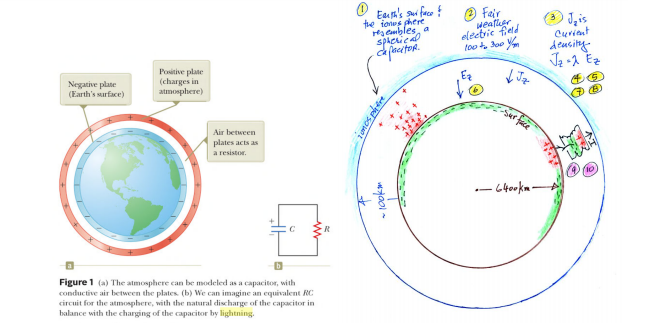
The Earth is essentially electrically neutral, yet a significant voltage difference arises between the upper atmosphere and the Earth's surface. This document provides extensive information regarding the potential difference and current density of the Earth. The illustration above demonstrates how the Earth's charges are arranged and interact.
The upper atmosphere contains electrically charged ions originating from the solar wind. This continuous influx of charged particles creates the potential difference on Earth, allowing charges to flow between the ionosphere and the Earth's surface via clouds.
According to discussions in the physics community, the Earth's surface is thought to carry a negative charge, while its overall net charge remains zero. Theories suggest that this negative surface charge may result from ionization at the Earth's inner core due to extreme temperature and pressure conditions.
Thus, the Earth generates a potential difference between its core and surface, with negative charges being driven towards the surface. This suggests the creation of a potential difference between the ocean floor and its surface, where negative charges from beneath the Earth rise up through the ocean, attracting positive ions from seawater (Na+, H+). This configuration positions the seafloor as the cathode of a colossal electrolysis cell, with the ocean surface acting as the anode.
Consequently, the ocean surface must be charged in relation to atmospheric clouds, as evidenced by lightning strikes. The National Oceanic and Atmospheric Administration states that while lightning strikes the ocean less frequently than land, when it does occur, the water acts as a conductor.
Nature's batteries at the bottom of the ocean!
This video explores the fascinating concept of the ocean's role in energy storage, showcasing its potential as an enormous battery system.
Could lightning be completing an electrolytic circuit between the ocean's surface and its depths? It appears likely, yet information on the potential gradient between the seafloor and the surface is scarce.
The implications of this theory suggest that our planet could be home to a vast electrolysis cell, with energy sources already at work in a somewhat chaotic manner. What if we could harness these elements into a cohesive system that consistently generates hydrogen fuel from ocean water as long as the Earth spins and the Sun shines?
Such a breakthrough could eliminate the need for fossil fuels, facilitating a rapid transition to a green economy. If human-induced CO2 emissions are a primary driver of climate change, this would offer a method to significantly reduce emissions while preserving economic stability. While the feasibility of this concept remains uncertain, the potential seems immense and warrants further investigation.
I plan to share more insights on hydrogen production from water, so please consider following my updates.
How to use the ocean as a giant battery
This video discusses innovative methods for leveraging the ocean's natural capabilities as a large-scale energy storage system, emphasizing its potential for sustainable energy solutions.